Introduction
Patellar tendinopathy (PT) is a clinical syndrome characterised by anterior pain of the knee, with focal tenderness; it is most often located in the lower pole of the patella in the proximal zone of the patellar tendon(1). These injuries are mainly caused by repeated movements that generate excessive loading of the tendon, particularly during sports activities characterised by rapid changes in direction, jumping and running, such as volleyball, basketball or soccer(2).
The incidence of PT within a sports season among a general population of athletes is estimated to be 14.2%, with a greater incidence in jumping sports. The maximum incidence is seen in volleyball players (up to 44.6%)(2). The condition affects men more than women (2:1.2 proportion). A detailed systematic review of the risk factors for PT showed anthropometric parameters such as a high body mass index, large waist circumference, discrepancies in the length of the extremities or a flat arch of the foot to increase vulnerability to such injuries(3).
The diagnosis is based on the anamnesis (case history) and physical examination, combined with complementary imaging tests particularly in the form of ultrasound and magnetic resonance imaging (MRI). Different methods have been described for treating PT(4). However, there is no agreement as to which management strategy is best, for despite the many treatment options, the current literature offers no clinical evidence on the superiority of one treatment alternative over the rest. The ideal management for PT therefore remains unclear(4,5).
The present study describes the different diagnostic options and conducts an analysis of the main current conservative and surgical management strategies.
Pathophysiology
A number of models have been proposed to explain the pathophysiology of PT. One of the most current proposals is the continuous model of Cook et al.(6), which postulates that a normal tendon can resist loading until the individual resistance threshold is exceeded. Once the elastic resistance of the tendon has been surpassed, reactive tendinopathy develops in a first phase, followed by tissue deterioration and posterior fibrillar degeneration.
According to Scott et al.(7), when the tendons are chronically exposed to loading volumes that exceed their physiological capacity, a cumulative injury and repair cycle takes place, ultimately leading to either damage or regeneration, depending on how the load is managed. In other words, if the tendon is given the necessary time to recover, with good local conditions of blood flow and nutrition, the healing machinery will prevail, with complete regeneration. However, if the recovery period is too short and/or the blood flow is inadequate, the repeated tension will lead to definitive tendon damage.
Biochemical studies warrant the idea that in the inflammatory process, attempted regeneration and posterior degeneration are interlinked - thus supporting the hypothesis that the pathogenesis of tendinopathy is a continuum extending from physiology to the manifest clinical presentation of disease(8).
Microdialysis techniques have been used to show that repeated mechanical stretching increases prostaglandin E2 (PGE2) production on the part of the fibroblasts of the human patellar tendon. This prostaglandin is a potent inhibitor of type I collagen synthesis, and exerts catabolic effects upon tendon structure, reducing proliferation and the production of collagen(9). In turn, a rise in lactate concentration is observed as a consequence of an insufficient vascular supply. This increase in lactate and tissue hypoxia in turn induces the production of vascular endothelial growth factor (VEGF). In addition to its angiogenic properties, VEGF is able to regulate the expression of substances that promote extracellular matrix degradation, thereby altering the mechanical properties of the tendons. This could predispose the tendon to recurrent microdamage, with spontaneous rupture over the long term. As a result of neoangiogenesis, nerves generally accompany the neovessels within the tendon. These data speak in favour of the hypothesis that neovascularisation is associated to the clinical symptoms, and in particular to pain(7,8,9).
In this phase, the tendon shows signs of degeneration and neovascularisation at ultrasound exploration, though the patient is generally asymptomatic. It must be taken into account that the metabolic rate of tendons is relatively low and lower than that of skeletal muscle (oxygen consumption is 7.5-fold lower, and the tendon collagen turnover time ranges from 50-100 days(10). Consequently, tendon recovery after damage takes longer than muscle recovery: while muscle damage takes weeks to recover, tendon injury takes months(3,4,6).
Diagnosis
The diagnosis of PT is based on a detailed case history, the physical examination, and the imaging test findings (musculoskeletal ultrasound and MRI).
Symptoms
Patients report pain well located within the lower pole of the patella and anterior zone of the knee. The pain is characteristically insidious at the start, being triggered by physical activity, though it gradually becomes more persistent as the frequency and intensity of exercise increase(11). In advanced stages it can even affect the activities of daily living of the patient (e.g., walking up and down stairs), with pain in response to prolonged periods seated, and can even cause sleep disturbances(12).
Physical examination
Quadriceps atrophy and hypotonicity are common findings in patients with long-evolving PT. Sudden and rapid contraction of the quadriceps with the knee extended can also trigger pain(11).
[[{"fid":"5589","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"1":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"1"}}]]
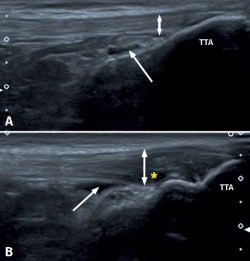
Distal PT in the adult (Figure 1) is also found as a complication of Osgood-Schlatter disease. This form of osteochondritis of the anterior tibial tuberosity manifests at prepubertal ages (males: 12-15 years; females: 8-13 years), it is produced by traction of the tendon upon its distal insertion in the distal tuberosity of the tibia, and is characterised by pain in response to palpation of the anterior tibial tuberosity in the physical examination(13). Its appearance has also been described at 12-18 months, before the completion of skeletal maturation(14).
It is important to mention the differential diagnosis of femoropatellar pain syndrome: although differentiation is easy in most cases, there are situations in which the conditions may coexist. Very useful functional tests are available and can be made with or without the help of functional bandages (McConnell). One way to reproduce the pain in the clinical examination is to perform a squat test(Figure 2). Squatting is performed with 30° of flexion with single-leg support on the explored leg, and with the contralateral knee in full extension(15). The test proves positive for PT if the increase in loading triggers pain, and we can also assess muscle strength and quadricipital and ischiotibial contraction(4).
[[{"fid":"5590","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"2":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"2"}}]]
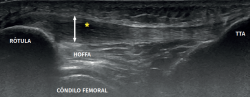
We can also discard the presence of intraarticular alterations of the knee (chondral or meniscal disease and Hoffa's fat pad syndrome, and referred pain of the hip(16) based on studies complementing the physical examination.
Classification
The Victorian Institute of Sport Assessment-Patella (VISA-P) is a very useful tool for assessing pain and function, and allows us to evaluate the severity of the symptoms and monitor the results(17,18).
In 1973, Blazina introduced the term jumper's knee to describe PT(19). This author developed a classification based on the clinical characteristics of patients with patellar tendinosis. The same classification is still used, with some minor modifications. It comprises four grades (with one sub-grade), according to the severity of the symptoms:
- Grade I: pain during sports activity.
- Grade II: pain at the start of sports activity, disappearing after warm-up, and reappearing at muscle fatigue onset.
- Grade IIIa: pain during and after exercise, but allowing sports activity.
- Grade IIIb: pain during and after exercise, not allowing sports activity.
- Grade IV: complete tendon rupture.
Tendinopathy is defined as acute (duration 1-6 weeks), subacute (6-12 weeks) or chronic (over 3 months).
Ultrasound diagnosis
Musculoskeletal ultrasound is very useful for exploring the tendon disease. With the introduction of high-resolution ultrasound systems, this type of exploration became crucial for the study of lesions of this kind(12,20). Ultrasound is non-invasive, inexpensive, rapid and dynamic, and allows us to obtain much information on the tendon. It is used both for diagnostic purposes and for guiding invasive therapies. Ultrasound is operator-dependent, however; the learning curve is therefore of vital importance for mastering the technique(20,21,22). Studies such as those of Wardeb et al. have evidenced the advantages of colour Doppler (CD) ultrasound versus MRI(20).
[[{"fid":"5591","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"3":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"3"}}]]
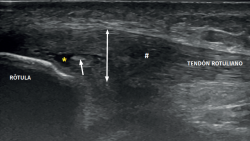
The ultrasound study of a damaged tendon evidences hypoechogenicity and loss of the fibrillar pattern, the appearance of disorganised connective tissue, and thickening of the tendon (Figure 3). In some cases we observe well delimited hypoechoic images indicating intra-substance rupture with nodular or fibrillar shapes, commonly manifesting in the deepest zone of the tendon in the lower pole adjacent to the patella (Figure 4).
[[{"fid":"5592","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"4":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"4"}}]]
In the middle portion of the tendon we may observe thickening (> 3.5 mm) associated to hypoechoic areas with zones of disorganised collagen and loss of the fibrillar pattern.
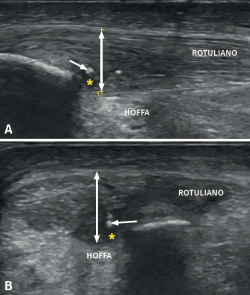
It is also common to identify cortical irregularities in the proximal enthesis of the tendon at the point where it joins the patella, involvement of the paratendon and adherences to Hoffa's fat pad. Any calcifications will be seen as white hyperechogenic zones (Figure 5).
[[{"fid":"5593","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"5":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"5"}}]]
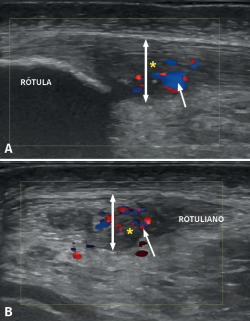
Colour Doppler ultrasound offers a further advantage in the study of tendinopathy, since it allows the visualization of blood flow within the tendon. Since a healthy tendon is practically avascular, the presence of blood vessels in the tendon is indicative of a disease process(20,23).
[[{"fid":"5594","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"6":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"6"}}]]
The appearance of intra-tendon vascular activity (Figure 6) detected by CD or Doppler-power (DP) ultrasound, interpreted as corresponding to neovascularization, has been described in different studies as part of a repair or repair failure process(24).
Magnetic resonance imaging diagnosis
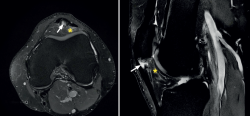
The most common MRI finding is increased signal intensity in the lower pole of the patella, with local widening of the affected zone of the tendon (Figure 7)(14,25). The most evident advantage of MRI with respect to ultrasound is its capacity to visualise associated lesions in the joint and cartilage, as well as the presence of bone edema and involvement of Hoffa's fat pad (Figure 7). As clear disadvantages, mention must be made of the high cost of the technique and the impossibility of performing exploration in motion or at different angles of flexion of the knee.
[[{"fid":"5595","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"7":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"7"}}]]
Treatment
Appropriate treatment of PT depends on the severity of the symptoms and on their duration over time. Conservative management is initially indicated, and if this proves insufficient, then surgery should be decided(4,5,25,26,27).
Conservative management
Conservative management includes noninvasive therapies such as the use of analgesics and antiinflammatory drugs, rehabilitation through physiotherapy (mainly eccentric exercises, as explained further below), and also invasive percutaneous techniques(4,28).
Analgesics and fundamentally nonsteroidal antiinflammatory drugs offer scant efficacy in the management of PT. This is all the more so considering that we generally treat advanced stages of the injury, with no active inflammatory process. Although widely used, corticosteroid infiltrations have been shown to have more risks than advantages. Their main risk is contribution to deterioration of the tendon tissue, with the possibility of rupture or calcifications, as well as the alteration of collagen synthesis(28).
Different rehabilitation programs involving physiotherapy have been described in the literature(29,30). At present, eccentric exercises have become established as the main conservative management option for PT(4). The most widely used eccentric exercises programs last 12 weeks and comprise 1-2 daily loading sessions that result in improvement of the pain and reduction of the neovascularization of the tendon(29,30). These programs are to be supervised by trained staff to determine the working range, the loads and the way of performing the exercises(31). A correct eccentric working program(29,31) associated to stretching and analgesic isometric exercises(32) affords very good outcomes when combined with other conservative therapies.
With regard to the invasive percutaneous therapeutic options, mention must be made of the ultrasound guided galvanic electrolysis technique (USGET), a minimally invasive procedure involving the application of galvanic currents to the damaged tissue, using a series of specific parameters. This technique produces a non-thermal electrochemical reaction that triggers an inflammatory and subsequently a regenerative process in the damaged tissue(5,33,34,35). The inflammatory and regenerative process induced by USGET must be complemented by eccentric exercises to stimulate the proliferative and remodelling phase, which in many cases leads to the healing of the tendon lesion(5). The outcomes of USGET have been described in the literature both over long periods of follow-up to assess the safety of the technique(34), and in the context of controlled trials(35).
High volume image guided injection (HVIGI) in turn targets neurovascular growth (from the paratendon to the tendon). This technique consists of high volume ultrasound-guided injections at the interface between the patellar tendon and Hoffa's fat pad (10 ml of 0.5% bupivacaine, 25 mg of hydrocortisone and 12-40 ml of physiological saline). The injections produce local mechanical effects, disrupting or occluding the neovessels and their accompanying nerve supply, and are mainly used in PT where conservative management proves insufficient and neovessels are identified by Doppler ultrasound(36).
In those cases where biological therapy with platelet rich plasma (PRP) is contemplated, the lack of established therapeutic protocols must be taken into account(4). There is much debate in the current literature, due to the presence of controversial results among the different studies, and there is little agreement on the number of infiltrations or the best concentration of platelets and leukocytes suited to each case(37,38,39). In the tendinopathy consensus of the European Society of Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA), PRP is regarded as a valid therapeutic option for PT. Infiltration of PRP is recommended within the tendon and guided by ultrasound. Some studies have reported better outcomes with two doses instead of only one, with improved responses in acute tendinopathy versus chronic disease. In turn, the joint utilization of local anaesthetics is not advised, because of their deleterious effect upon platelet aggregation. Lastly, the results are reportedly better when PRP is used together with rehabilitation protocols characterised by good patient adherence (5). The future of PRP is still open, and further studies are needed, with specific standardised protocols for the preparation of PRP in application to acute and chronic lesions.
Other conservative treatments such as the use of progenitor cells, gene therapy or the use of biomaterials are being considered as future options that nevertheless require further studies and improved knowledge(5).
Surgical treatment
In the presence of persistent pain and functional impotency limiting sports activity despite conservative management, the latter is considered to have failed, and in such cases more invasive treatment is indicated. Although no defining comparative studies have been made to determine which surgical technique is best, the current tendency is to use arthroscopy combined with ultrasound (sonosurgery), as described by Alfredson et al.(5,25,36,40,41,42). Debridement is carried out of the deepest and most deteriorated part of the tendon, where paratendon and Hoffa's fat pat adherences form (arthroscopic debridement), allowing better control of depth and orientation of triangulation(25).
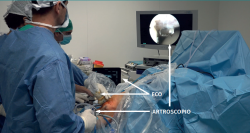
The patient is placed in supine decubitus, and an arthroscopic exploration is made to analyse possible lesions associated to PT. The surgeon must have direct vision on the ultrasound system and arthroscopy tower (Figure 8). Two portals are used - anterolateral and anteromedial - located slightly lower than when used to perform meniscectomy. Ultrasound-guided debridement of Hoffa's fat pad adjacent to the paratendon is carried out. It is advisable not to use a tourniquet, in order to allow ultrasound visualization of hypervascularization zones and their elimination using a synoviotome. In the presence of intra-substance rupture, careful debridement is required, with removal of the possible distal osteophyte in the patella.
[[{"fid":"5596","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"8":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"8"}}]]
Other surgical techniques are available, such as combing of the tendon, open osteotendinous resection, arthroscopic tendon resection, open tendon resection-repair or ultrasound-guided electrocoagulation(27,41,42). At present, the European consensus suggests the use of sonosurgery to treat refractory PT(5).
Conclusions
Patellar tendinopathy remains a challenge for the sports traumatologist. Correct classification and the use of ultrasound for diagnosis, treatment and follow-up are crucial. In cases unresponsive to conservative management, the surgical option with sonosurgery offers good outcomes.