Introduction
The treatment of joint cartilage defects constitutes one of the main challenges in current orthopedic surgery. The reported incidence of chondral lesions among individuals between 40-50 years of age is 60%(1). These defects give rise to alterations that may result in considerable pain and functional limitation, and moreover pose a risk of osteoarthrosis over the long term(2,3). In addition, it has been seen that such patients present clinical situations similar to those of individuals with osteoarthrosis in wait of total knee replacement surgery, with even poorer clinical scores than those in wait of anterior cruciate ligament reconstruction(4). All this is attributable to the high specialization of cartilage tissue, allowing correct load transmission and affording a uniform joint contact surface. Nevertheless, repair potential is very limited, due to the avascular nature and low mitotic activity of the chondrocytes(5).
The aim of most treatments is to create a congruent joint surface with competent biomechanical properties, restore joint function, and prevent degeneration to osteoarthrosis. Such surgical treatment methods include repair techniques, regenerative procedures and palliative techniques(6). Repair techniques involve the use of tissue histologically different from cartilage, while regenerative procedures are characterized by complete replacement of the damaged tissue, maintaining the same architecture and biomechanical properties.
The repair techniques include bone marrow stimulation strategies, which have been in use since 1959, when Pridie(7) introduced subchondral perforation surgery. This technique was subsequently improved upon by Steadman(8) with microfracture surgery, in which motor-driven perforation was eliminated in order to avoid heat-induced necrosis. This technique exposes the chondral lesions to mesenchymal stromal cells ( MSCs) from the bone marrow. This exposure to cells and growth factors forms a clot that induces lesion repair(9). An important element introduced by Steadman is the correct preparation of the damaged zone through curettage of the calcified cartilage layer and removal of all the pathological cartilage tissue to the transition to healthy cartilage, in order to secure a bed better able to accommodate the mentioned clot.
At present, the nanofracture technique has been developed thanks to improved understanding of the architecture and function of subchondral bone. The mentioned technique uses a smaller diameter needle allowing better penetration of the subchondral bone, without compacting the margins of the perforations. This favours greater preservation of the trabecular structure, with posterior remodelling more similar to native subchondral bone(10,11). In addition, in vivo studies have shown that the histological qualities of the cartilage obtained are more similar to hyaline cartilage than those resulting from microfracture surgery(12,13).
Behrens(14,15) introduced autologous matrix-induced chondrogenesis (AMIC) - a technique that combines microfractures with the application of a biodegradable type I/III collagen membrane that stabilizes and protects the clot formed by the microfractures. The matrix or scaffold is affixed to the cartilage defect by means of sutures or fibrin glue. This procedure can be carried out via a miniarthrotomy or adopting a fully arthroscopic approach, and moreover offers the advantage of requiring a single surgical step.
In recent years there has been an increase in the number of AMIC-like procedures, with variations in the surgical technique and type of matrix employed. There are promising lines of research on the addition of growth factors to the matrix, resulting in selective recruitment and stimulation of the mesenchymal cells(16,17,18). Such factors moreover could activate the chondrocytes surrounding the healthy cartilage to fill the osteochondral defect. The purpose of the present study was to review the underlying biological principles, the surgical technique, and the current clinical status of matrix-induced chondrogenesis.
Basic science
Mesenchymal stem cells, MSC, are progenitor cells found in a great variety of adult tissues, including the bone marrow(19). These cells are able to differentiate towards the formation of mesodermal tissues, including cartilage, bone and adipose tissue(20). In order for this to occur, however, correct interaction among the MSCs, the extracellular matrix (ECM) proteins and growth factors (GFs) is essential(21). Tallheden(22) observed that MSCs from microfractures exhibited the same phenotypic plasticity as the chondrogenic cells of the basal substrate. Kramer in turn demonstrated that collagen matrixes satisfactorily house the cells from the bone marrow, since he was able to isolate MSCs from the matrixes(23).
[[{"fid":"4528","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"1":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"1"}}]]
An ideal matrix is able to imitate the biology, architecture and mechanical properties of native cartilage, with sufficient structural strength to support the mechanical requirements of the joint. It must prove stable in the tissue environment and allow cell migration and adhesion, as well as proliferation and differentiation. Furthermore, the material must be biodegradable and undergo remodelling while the new cartilage is formed and replaces the original structure, without releasing harmful degradation products and without stimulating an inflammatory response that could prove negative for tissue regeneration(24,25).
Although the AMIC technique was originally described with the use of a type I/III collagen membrane(15) (Chondro-Gide®, Geistlich Biomaterials, Wolhausen, Switzerland), different matrixes comprising different materials have been used for this procedure. These implants may be made of natural or synthetic polymers, as well as of mixed compounds (Table 1). The most widely used natural polymers include collagen, hyaluronic acid, chitosan (Figure 1), agarose and alginate, and are characterized by great biocompatibility and biodegradability. Likewise, their surfaces contain ligands that are recognized by cell receptors, thereby facilitating adaptation(26). On the other hand, synthetic polymers such as polylactic acid or polyglycolic acid are characterized by mechanical resistance, a lack of immunogenicity, and a zero risk of pathogen transmission(25,26).
[[{"fid":"4529","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"2":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"2"}}]]
Type I/III collagen-based matrixes are the most widely used option, and are cited in a greater number of publications(27,28,29,30). These matrixes are structured as a double layer, with a porous surface allowing MSC fixation to the collagen fibers for cell proliferation and differentiation, and a non-porous smooth surface that retains the clot within the defect. This type of matrix is reabsorbed within 6-24 weeks after implantation. Gille(31) demonstrated in his study that the cells that develop in the membrane form a multilayer structure with cells similar to chondrocytes. Gigante(32) obtained biopsies from 5 patients previously subjected to treatment for chondral lesions and recorded a mean histological score (International Cartilage Repair Society [ICRS]) of 60 - where a score of 100 indicates maximum similarity to joint cartilage - and also described an arthroscopic visual appearance similar to that of healthy cartilage.
Absorbable polyglycolic acid (PGA) matrixes treated with hyaluronic acid are also available, acting as a sponge, retaining the clot and MSCs within the chondral defect(33). Animal models have shown that hyaline cartilage of greater quality is formed with the use of this type of implant than with microfracture surgery alone(34,35); likewise, hyaluronic acid has been seen to induce chondrogenic differentiation of the MSCs(36,37). The mechanical stability of this matrix allows for easy handling during surgery and safe fixation with both sutures and fibrin glue, and reabsorbable pins. To date, its presence in the literature is limited to case reports(33,38) and case series involving few patients(39,40).
Implants composed of semisynthetic derivatives of hyaluronic acid can also be found, structured into randomly distributed fibers, with no particular predominant direction. This facilitates cell contact and extracellular matrix formation, as well as MSC retention. Buda(41) described their use in 2010, combining the AMIC technique with bone marrow MSCs obtained from the centrifugation of iliac crest marrow aspirate and platelet rich plasma (PRP). These membranes are impregnated with the MSC concentrate and are placed in the defect, followed by the application of PRP supplying growth factors. Vannini(42) reproduced the technique in 6 patients with osteochondritis dissecans, with good results.
Surgical technique
The original surgical technique was described by Benthiem and Behrens(14,15) in application to chondral defects of the knee. The indications of the technique are chondral or osteochondral lesions measuring under 1.5 cm2 in size. In the case of osteochondral lesions, the authors filled the bone defect with cancellous bone from the proximal tibia, compacting it and placing the membrane upon the grafted bone. The contraindications of the technique are mirror lesions or lesions of an inflammatory origin; damage to the collateral ligaments or cruciate ligaments; deficits in extension of over 10º or in flexion of over 100º; poor alignment of the lower extremity; and associated fractures.
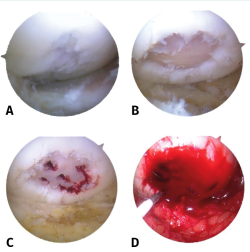
The procedure can be performed under spinal or general anesthesia. The patient is placed in supine decubitus, and the use of tourniquet ischemia is advised. In a first step, arthroscopy of the affected joint is carried out to check the size and location of the defect, and to diagnose possible associated lesions. If the criteria for AMIC are met, the authors recommend a minimally invasive arthrotomy. Under direct visualization of the lesion, curettage of the defective cartilage tissue is performed until healthy cartilage is reached, with removal of the layer of calcified cartilage until subchondral tissue appears. The microfractures technique is then carried out as described by Steadman(8).
Once this process is completed, the final size of the chondral defect is determined to calculate the dimensions of the membrane to be placed. It is advisable to use a template to transfer the correct size to the matrix. Some surgeons use aluminium or plastic sheets for this purpose. The authors suggest the choice of a size slightly smaller than that of the defect, in order to avoid its displacement with joint motion.
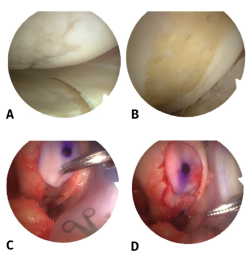
The next step involves membrane fixation. The fixation method preferred by Behrens comprises the use of partially autologous fibrin glue obtained from the centrifugation of peripheral blood of the patient, and mixing it with exogenous fibrinogen. It is also possible to use commercial fibrin formulations. It is advisable to position the membrane slightly below the level of the cartilage in order to avoid posterior displacement (Figure 2).
[[{"fid":"4530","view_mode":"default","fields":{"format":"default","alignment":""},"type":"media","field_deltas":{"3":{"format":"default","alignment":""}},"link_text":null,"attributes":{"class":"media-element file-default","data-delta":"3"}}]]
With regard to postoperative management, some authors recommend knee immobilization during 7 days in extension(43). Others keep the operated extremity without weight bearing for a period of 2-6 weeks(41,42,43,44). Most surgeons allow impact-generating activities after 6 months and sports activities from one year after surgery. The authors describing the original technique allowed maximum partial weight bearing of 15-20 kg during 6 weeks. Furthermore, maximum flexion of the knee is limited to 30º during two weeks, with progressive increments to 60º and 90º from week 4 and 6, respectively, with the incorporation of joint range and muscle strengthening exercises.
Purely arthroscopic techniques have been described in recent years. In 2012, Piontek(45) described a variation of the technique in which curettage is followed by the use of trephines to determine the size of the lesion. Subsequently, under dry arthroscopy conditions, subchondral perforations are made, followed by the placement of a Chondro-Gide® membrane in the defect. Lastly, the membrane is covered with commercial fibrin glue (Tissucol®, Baxter, Warsaw, Poland), and stability is checked by performing flexion-extension movements. The joint range over which the lesion remains without bearing weight is then recorded to determine the safe mobility range for rehabilitation. In order to avoid displacement of the membrane, drains are not used.
In 2015, Sadlik(46) described an arthroscopic technique for the treatment of patellar lesions. A retracting plate is used, bound to extra-articular sutures, and placed under the medial patellar retinaculum - allowing elevation and inclination of the patella in order to better visualize the lesion and maintain the operating space in the dry step of arthroscopy. In addition, a guide and introducer are used to insert the matrix and position it correctly over the chondral defect.
Schagemann(47), in a study of 50 patients, compared the arthroscopic technique versus the miniarthrotomy procedure, and concluded that there are no significant differences between them in terms of the outcomes. However, the decision to choose one technique or the other should be based on surgeon skill.
Different arthroscopic techniques have been proposed to treat chondral lesions in other joints such as the ankle(48,49), hip(50) and shoulder(51).
Clinical evidence
Buda(41) performed the AMIC technique in 20 patients using Hyalofast® together with MSCs and PRP, and recorded statistically significant improvements in the International Knee Documentation Committee (IKDC) score and Knee Injury and Osteoarthritis Outcome Score (KOOS). At magnetic resonance imaging (MRI) controls after 12 and 24 months, improvements were recorded in the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score, and a statistically significant relationship was observed between the KOOS at 24 months and MRI signal intensity. The histological study revealed a matrix rich in proteoglycans, an almost regular surface layer with the presence of cells uniformly distributed within the tissue, and type II collagen positivity throughout the thickness of the biopsy sample.
Vannini(42) used the same technique in 6 patients between 14-18 years of age with osteochondritis dissecans and a mean defect size of 4.6 ± 1.5 cm2. The same satisfactory clinical outcomes were obtained, with an increase in IKDC score, but no significant correlation was observed between the MOCART parameters and the clinical scales.
In 2012, Kusano(27) published the results corresponding to 38 treated patients with single lesions over 2 cm2 in size and aged under 50 years, using Chondro-Gide®. Significant increases were recorded in the IKDC and Lysholm scales, but the MOCART score was not correlated to the clinical outcomes, since most of the lesions showed incomplete defect repair, with damaged repair tissue and a heterogeneous structure, as well as an altered subchondral plate.
Pascarella(52) modified the technique seeking to increase the MSC load. Perforations were made instead of microfractures, and the Chondro-Gide® matrix was impregnated with bone marrow concentrate obtained directly from aspiration through one of the perforations. The clinical outcomes (IKDC and Lysholm scales) revealed increases similar to those obtained by Kusano(27), and the postoperative MRI study showed a significant decrease in the area of the defect and in subchondral edema in 53% of the patients.
In 2010, Gille(43) published the results of 32 chondral lesions treated with type I/III collagen membranes in 27 patients between 16-50 years of age, with a mean defect size of 4.2 cm2 and subjected to a minimum follow-up of two years. A significant increase in the Lysholm score and in the ICRS scale was observed during the first 24 months of follow-up, with a decrease from two years onwards. In 2012(28), as part of the AMIC Registry, the sample was expanded to 57 patients, with the observation of a significant increase in the Lysholm score and in the pain visual analogue scale (VAS).
A more recent study involving 21 patients with a mean follow-up period of 7 years recorded a significant increase in the IKDC and Lysholm scores throughout follow-up(53). Furthermore, no association was observed between patient age and body mass index (BMI) and the functional indices. A decrease in the area of the defect and in subchondral edema was also recorded in 66% of the patients.
With regard to ankle joint lesions, significant improvements have been observed in the VAS score and in the ankle score of the American Orthopedic Foot and Ankle Society (AOFAS), in the studies published by D'ambrosi(54,55), Usuelli(48) and Valderrábano(56,57). Likewise, MRI assessment of the cartilage revealed an increase in the MOCART score(48,56).
A number of studies have reported improvements in the modified Harris Hip Score (mHHS) in chondral defects of the hip(58,59,60). Fontana(58) compared the AMIC technique with microfracture surgery in acetabular lesions caused by femoroacetabular impingement (FAI), and found the clinical improvement to remain stable over the 5 years of follow-up, while in the microfractures group it was seen to gradually worsen.
Discussion
Matrix-induced chondrogenesis is a treatment option for cartilage injuries, involving the placement of a membrane capable of containing and stimulating MSCs to produce cartilage tissue. The systematic reviews carried out by Shaikh(61) and Gao(62) highlight the few existing high-quality randomized studies, due mainly to the limited number of patients and shortcomings referred to the duration of follow-up and study design. This is particularly manifest in the case of those studies that establish comparisons with other repair techniques. No clinical trials to date have compared AMIC versus autologous chondrocyte implantation (ACI) in the knee or ankle, and likewise no comparative trials have been made with microfracture surgery in the latter.
In contrast, randomized studies have compared AMIC versus microfracture surgery alone(63,64). Anders(64) compared 38 patients with lesions presenting a mean size of 3.4 cm2 and 37 years old on average, with a follow-up of 2 years. A significant increase was observed in ICRS scale and in the modified Cincinnati scale for both microfractures and for the AMIC group at one year of follow-up, with maintenance of the increase after two years. There were no significant differences between the groups. Volz(63) recorded similar results after two years. However, after 5 years there was a significant decrease in the clinical outcomes in the microfractures group, while the patients in the AMIC group maintained or even improved their outcomes.
The ACI technique involves the harvesting of cartilage samples from a non-weight bearing or chondral lesion donor zone, followed by processing in the laboratory to release and culture the chondrocytes. In second step surgery, these chondrocytes are implanted in the defect. The third generation of this process - matrix-induced autologous chondrocyte implantation (MACI) - includes the use of a membrane to retain the chondrocytes within the lesion. To our knowledge, only one study has compared AMIC versus MACI to date(60). In the mentioned study, Mancini(60), in patients with hip lesions measuring 2-4 cm2 in size, compared AMIC versus MACI and concluded that both groups showed clinical improvement, though without significant differences over the 5 years of follow-up. However, the study lacked a randomized design and did not contribute MRI data through MOCART.
The literature does offer high-quality studies establishing comparisons with other techniques such as microfracture surgery(65,66) and mosaicplasty(67). From the results obtained, it can be deduced that the AMIC technique yields results similar to those of ACI, with the advantage that AMIC requires only single surgery, and the structure and logistics of a laboratory are not needed.
Because of the relative novelty of this treatment, few studies reporting long-term outcomes are available. The identified studies with the longest follow-up involved a period of 5 years(43,56,58,60). Gille(43) reported deterioration of the clinical outcomes after 18-24 months as assessed by the ICRS, Lysholm and Tegner scales. Anders in turn reported a tendency towards stabilization of the clinical outcomes from 12 months, and 11% of the patients even experienced a worsening of the clinical parameters. However, Fontana(58) and Mancini(60) recorded good results that persisted over the 5 years of the studies - though all the patients had undergone additional operations for the treatment of femoroacetabular impingement (FAI).
A number of studies have included additional surgeries associated to treatment of the chondral lesions, such as realignment osteotomies(27,41,43,56,68) or the treatment of femoroacetabular impingement (FAI)(58,60) - making it difficult to determine whether the clinical effect is a result of repair of the cartilage defect, the associated surgeries, or both. Only Kusano(27) compared patients treated exclusively with the AMIC technique versus patients in which realignment osteotomy was added. No significant differences in outcome were observed. Because of this variability in terms of the associated treatments, it is difficult to compare the rehabilitation protocols and determine which is best.
To date, no studies have reported serious adverse effects related to this technique. With regard to repeat surgeries, these have been limited to the elimination of intralesional osteophytes(29,68), often in relation to the microfracture procedure.
In this regard, several microfracture techniques have been used as a first step for AMIC. These range from the use of punches(27,43,58,60,64) to driving motors(56,58), though no comparative studies have established which approach is best for performing microfractures in the context of AMIC. Nevertheless, increased quality cartilage has been recorded with the use of nanofractures versus microfractures in studies conducted in vivo(12,13).
Another key technical aspect is matrix fixation. This can be done using sutures, fibrin glue or subchondral anchoring. Sutures and fibrin afford sufficient membrane stability(69), although the use of sutures is not only technically more demanding but also causes local damage to healthy cartilage similar to that seen in the early stages of osteoarthrosis(70). More recent studies show subchondral anchoring to offer the greatest mechanical resistance(71). The studies of Volz(63)and Anders(64) compared two groups of patients subjected to suture fixation versus another group in which fixation was made with fibrin glue - no significant clinical or radiological differences being found between them.
With regard to matrix composition, Welsch(72) found the repair tissue to be better with collagen matrixes than with matrixes based on hyaluronic acid, according to radiological criteria. This is in contrast to studies suggesting that hyaluronic acid is able to induce chondrogenesis(73,74). Wu(74) showed the addition of hyaluronic acid to polylactic-co-glycolic acid (PLGA) matrixes to yield greater levels of type II collagen and glycosaminoglycans. Mechanically, polyglycolic acid (PGA) matrixes have been shown to support greater loads, independently of the fixation used, compared with collagen membranes and PLGA membranes(31).
On the other hand, in a recent systematic review, Gao(75) concluded that there is not enough evidence that biomaterials composed of type I/III collagen can by themselves induce chondrogenesis. Indeed, in vitro studies indicated that type II collagen matrixes induce greater cell proliferation and a greater accumulation of collagen and glycosaminoglycans than type I collagen matrixes(76).
Conclusions
Matrix-induced chondrogenesis is a safe, technically reproducible and effective technique for the treatment of cartilage injuries, with good clinical outcomes for the patients. Furthermore, it has the advantage of being applicable in a single surgical step and without the morbidity associated with the creation of a donor zone - in contrast to ACI or mosaicplasty. Nevertheless, randomized studies involving larger patient samples and improved methodological designs are needed to establish comparisons with the rest of the existing therapeutic options. Likewise, long-term studies are required to determine whether the technique is able to avoid or delay joint degeneration.